Electron Groups Could Be Considered Which of the Following
Three or more unshared electrons C. A group of electrons can be a single bond double bond triple bond or a lone pair of electrons.
7 6 Molecular Structure And Polarity Chemistry
Please contact the person in charge at each of our group companies for consultations regarding business such as our products and service.
. O A double bond A single bond All of the proposed answers Alone pair of electrons Question 2 What is the molecular geometry if you have 3 single bonds and one lone pair around the centralatom. B A duet is a stable electron configuration for helium. Closer to H because Hydrogen has a lower electronegativity than Chlorine d.
Unshared pair of electrons are generally termed as lone pair of electronsin an atom which are. An inadequate model since the bond is ionic. Electron groups could be considered which of the following.
E All of the above statements are true. Trigonal pyramidal Tetrahedral O trional planar Bent. The effect of the electron-withdrawing NO 2 group is to decrease electron density at the ortho and para positions of the ring.
Which of the following is considered a single electron group. So in an empirical formula ratio of molecules cannot have a common factor. A double bond 4.
The electron pair in a H - Cl bond could be considered. A triple bond 2. The term valence electron is primarily introduced in grade 8th however students get to know details about it in higher grades.
Which of the following elements would lose an electron easily. A single bond 5 All of the above. In the Standard Model of particle physics electrons belong to the group of subatomic particles called leptons which are believed to be fundamental or elementary particles.
Valence electrons are considered to be one of the basic concepts that students must learn about in order to understand difficult concepts of Chemistry. What are considered electron groups. Option D B.
Electron groups could be considered as Lone pair electrons and bonded pairs of electrons. Lone-pair electrons Bonded pairs of electrons What are the groups of elements called that have very regular electron configuration They are considered the. A Na2 b Mg2 c AI2 d Si2 e p2 f s2 g F2 h Ar2 40.
Bonding electrons which are shared by a pair of atoms and nonbonding electrons which belong to a particular atom but do not participate in bonding. Question 1 Which of the following is considered an electron group to determine the electron group cometry. A An octet is when an atom has 8 valence electrons.
Electrons have the lowest mass of any charged lepton or electrically charged particle of any type and belong to the first-generation of fundamental particles. The valence electron for the main group elements exists only. A lone pair of electrons 3.
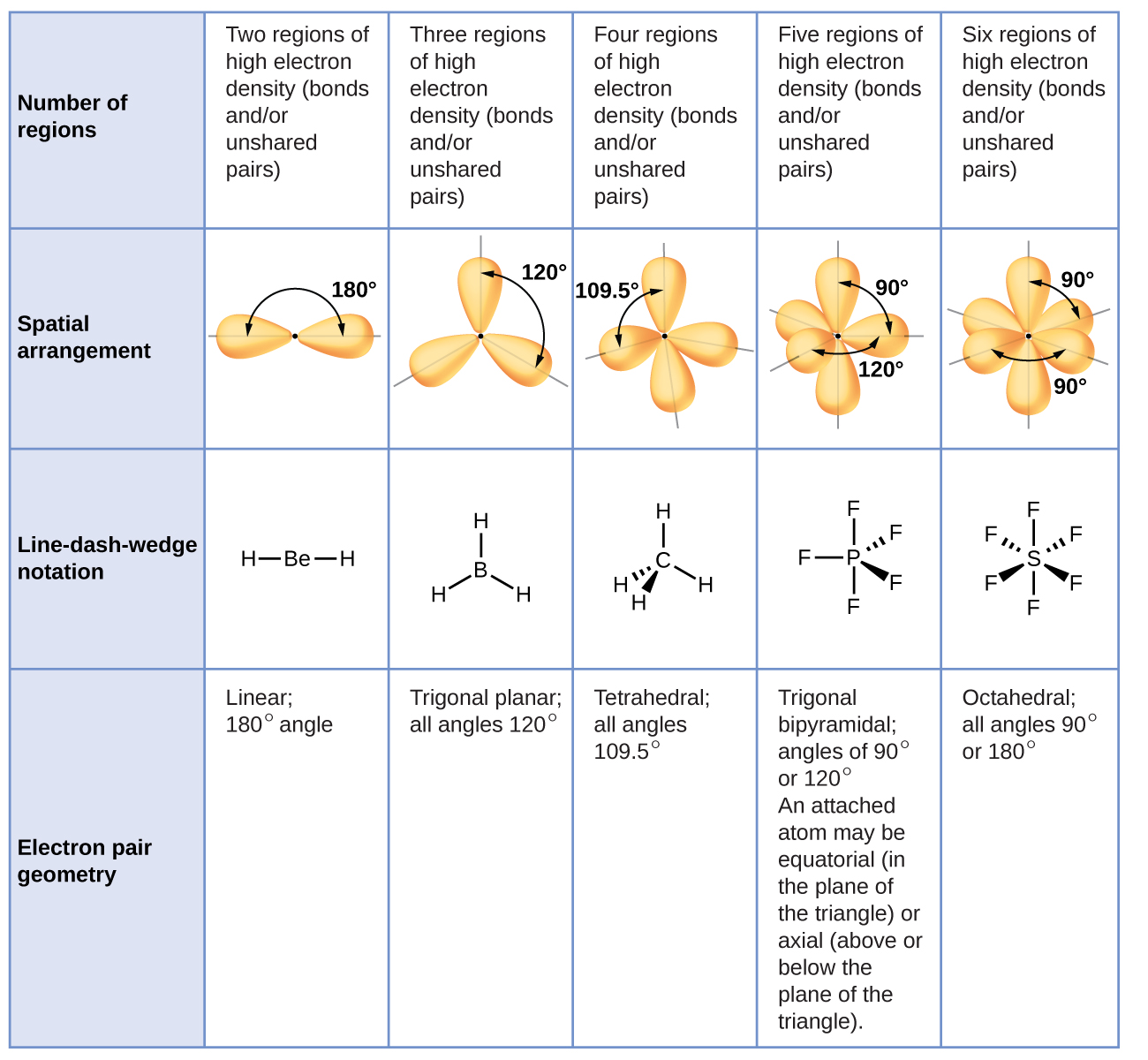
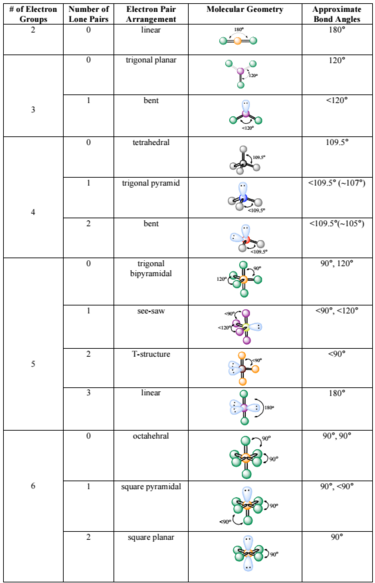
In CCl 4 the central carbon atom has four bonding groups of electrons. Bonded pairs of electrons Lone-pair electrons. Predict the valence electron molecular orbital configurations for the following and state whether they will be stable or unstable ions.
Closer to Cl because Chlorine has a higher electronegativity than Hydrogen c. What are the groups of elements called that have very regular electron configuration They are considered the. D A covalent bond occurs when electrons are shared.
नमनलखत म स कन-स ततव आसन स इलकटरन नह खत ह. According to this principle electrons are filled in the following order. C An ionic bond occurs when electrons are transferred.
The details and personal information of the whistleblowing shall be viewed by the person in charge of the compliance department of Tokyo Electron Ltd which operates the Supplier Hotline The. Correct option is C Empirical formula is the simplest ratio of molecules present in a compound. Molecular shape is determined by the number of electron groups around a central atom where a group consists of any number of electrons that occupy a _____ region around an atom.
Chemistry 1 Answer Dave Meave60 Apr 24 2018 Electron groups are lone pairs andor bonds since we explain bonds as a pair of shared electrons. Notice that there are two kinds of electron groups in this structure. Each chlorine atom has three nonbonding pairs of electrons.
The table below indicates the Molecular Geometry of the central atom depending on whether the groups of electrons around it are covalent bonds to other atoms or simply lone pairs of electrons. Possible five electron groups. Predict the valence electron molecular orbital configurations for the following and state whether they will be stable or unstable ions.
Actually not the one you might see most of oneness probably out of. The electrophile reacts preferentially at the meta position since those are the most electron-rich positions. The two or more electrons can be bonded by single bond double bond covalent bond of electrons can simply be lone pair of electrons.
All electron-donating groups behave similarly. Bonded pairs of electrons. Electron groups could be considered.
Which of the following elements would lose an electron easily. 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p The order in which electrons are filled in atomic orbitals as per the Aufbau principle is illustrated below. Any atom thats an ion D.
Five A literal groups has made full uh x two e two formulas or a exim uh except me in for most but the one you probably will see the most or the one is dead. Most electron-withdrawing groups behave similarly. IF the electron groups are covalent bonds.
For NF3 the Lewis Structure will give you something like Nitrogen in the center with 3 bonds to F atoms and 1 lone pair I dont know how to draw structures on here. Closer to H because Hydrogen has a larger radius and thus exerts greater control over the shared electron pair b. A double bond contains _____ electron pairs but is considered _____ electron group because these electrons remain near each other.

No comments for "Electron Groups Could Be Considered Which of the Following"
Post a Comment